By Russel Barsh, director of KWIAHT.
Over the past decade, San Juan County agencies and environmentalists have shifted public attention from reducing our use of biocides (pesticides, herbicides) to reducing our use of plastics, starting with single-use items such as straws, cups and bags.
This shift in focus is part of a wider national trend that benefits biocide manufacturers such as Dow and Bayer. We continue to spray toxic chemical compounds on our lawns, gardens, home foundations and ponds; and while most of these toxic products are sold in disposable plastic containers, we behave as if the environment is more threatened by empty bottles than by the poisons many of them once contained.
Meanwhile, images of rafts of floating plastic bottles have recently been superseded by revelations about the ubiquity of “microplastics,” and reports that they have been found in breast milk, blood and human brains. How concerned should we be?
To begin, plastics in most widespread use such as polyethylene (PE), polypropylene (PP), and polyester (PET) are not toxic or “poisonous” as such. Most of them break down eventually in the environment into carbon dioxide and water. Health issues with plastics are either mechanical (gastrointestinal blockage in animals that selectively consume plastic debris, for example) or associated with toxic compounds added to plastic products to make them more attractive (plasticizers, colorants, fragrances), sold or stored inside plastic containers, or adsorbed to plastic debris from the environment.
The National Oceanic and Atmospheric Administration defines “microplastics” as particles that range from 5 millimeters (about a quarter of an inch) down to one nanometer, which is smaller than a single protein molecule inside a living cell. Most microplastics can only be seen through a microscope, and are the result of abrasion and disintegration of larger plastic objects. The largest source of microplastics in our oceans appears to be washing, fraying and disposal of plastic (nylon, polyester) clothing, rather than plastic bags, bottles or straws.
How do microplastics get inside breasts or brains? They must be small enough to squeeze between cells in the walls of blood vessels: less than a thousandth of a millimeter (one micron), or one-sixth the size of a human red blood cell. Smaller particles may squeeze between the phospholipid molecules that comprise cell walls. Microplastics in this size range are often described as “nanoplastics.”
Nanoplastics are not the only extremely small particles to which our stomachs and lungs are routinely exposed. “Particulates” are micron-scale bits of matter. Many particulates are of natural origin, such as mineral dust from sand and soils, water droplets and tiny bits of decaying animals and plants. Soot from burning fossil fuels, dust from manufacturing and, yes, fragments of decomposing plastic products are also part of the particulate burden of the air we breathe and the water we drink. Reducing particulates in the air and drinking water has been a responsibility of the Environmental Protection Agency since it was established by Congress in 1972.
Are plastic particulates a greater danger to human or wildlife health than nonplastic particulates? The science on this question is complicated and much remains uncertain. Natural as well as artificial particles smaller than cells have been associated with reduced heart and lung functioning. In addition, particulates attract and adsorb oily chemical compounds from the environment that can include biocides as well as industrial waste, pharmaceuticals and detergents. Hence particulates can physically damage organs and cells, and they can also accumulate and transport chemical pollution, delivering toxic compounds to the stomachs, lungs and bloodstream of wildlife and humans.
Particulates composed of quartz sand, glass, metal, rubber and plastics differ in what kinds of compounds they adsorb best. Oily PCBs adsorb strongly to plastics, as do many pesticides in current widespread use. Artificial rubber (think automobile tire dust) also strongly adsorbs oily contaminants, which is one reason that road runoff can be so harmful to lakes and wetlands: it is rich in rubber particulates coated with oils and toxicants associated with burnt motor-fuel such as PAHs.
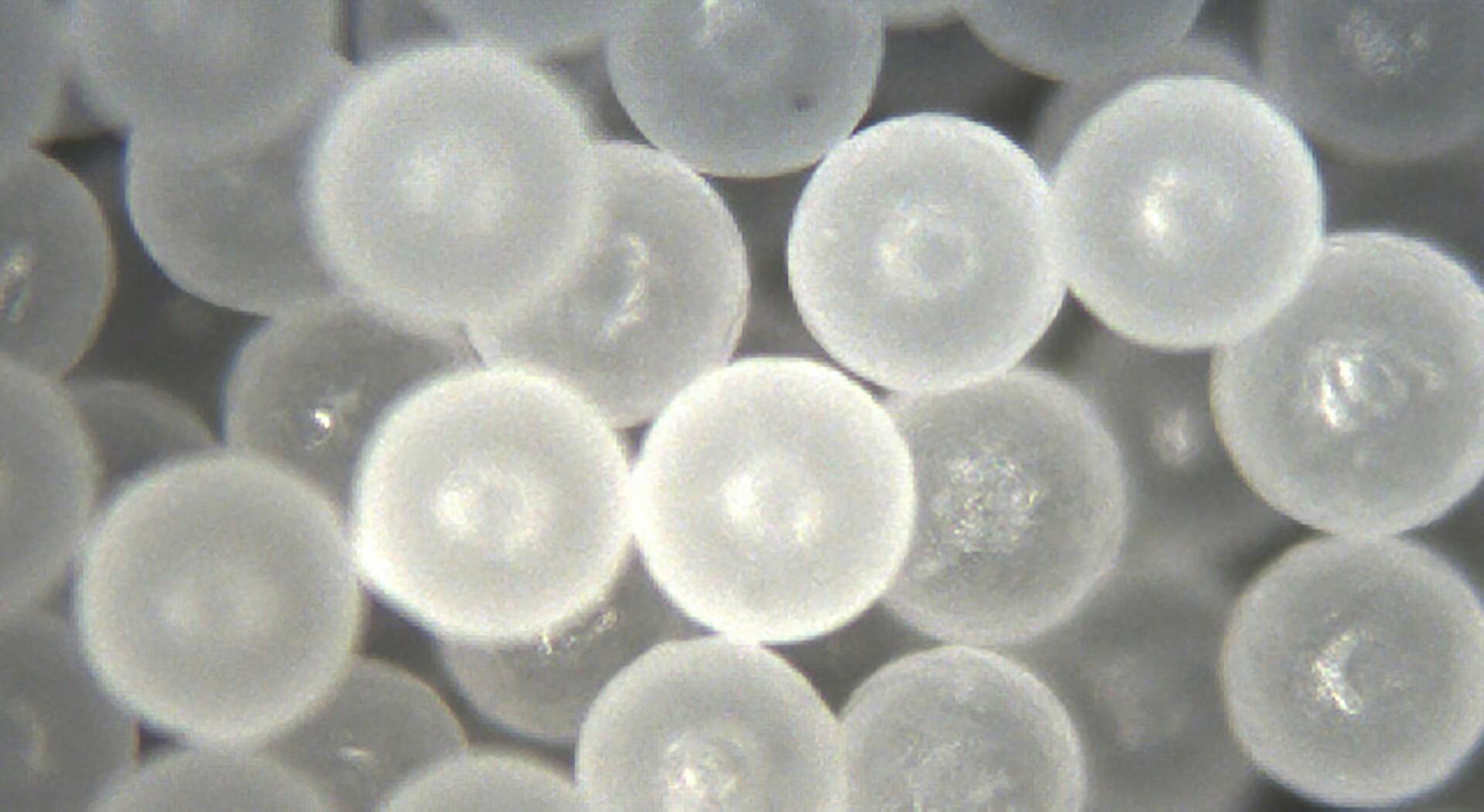
And here is where the microplastic story takes an especially surprising twist. There is a whole industry of making customized particulates, many of them plastic, for use in consumer products.
These so-called “microparticles” are manufactured to help spray, spread, and deliver compounds that are not soluble in water. Microparticles are coated or filled with the active ingredients in products as diverse as cosmetics, processed foods and pharmaceuticals, as well as pesticides and herbicides. Surfactants are routinely added to help keep the microparticles suspended and evenly mixed in the liquid or other matrix in which the active ingredients are delivered. Microparticles and surfactants are classified in the United States (but not in Europe) as “inactive” ingredients, and as such they do not need to be declared in product labeling.
Microparticles used in industry and medicine vary in composition, but many are made of plastics such as polystyrene. Plastic microparticles in personal-care, home and garden products may end up in the air and water. The widespread use of microparticles in products such as cosmetics and “meds” also helps explain why plastics are being found in human blood and organs. Reports of microplastics in the human body do not always distinguish between post-consumer debris, such as microfibers from synthetic fabrics, and the microparticles used in products that we deliberately ingest or smear on our bodies.
We should pay more attention to the fates of microparticles in our bodies and the environment. But let’s not forget that the potential health impacts of microplastics, including microparticles, are chiefly a result of the biocides we continue to apply to our homes, gardens and backyards. Reducing the outdoor use of biocides, and their “collateral damage” on nontarget organisms, should be our priority.